Answer
ΔH for the reaction is +12.2 kJ
Step-by-step explanation
Given:
The given reaction is:
The following are the given data:
What to find:
To calculate the ΔH for the given reaction.
Step-by-step solution:
Step 1: Multiply (a) through by 2
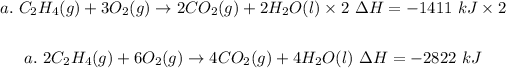
Step 2: Reverse (b)
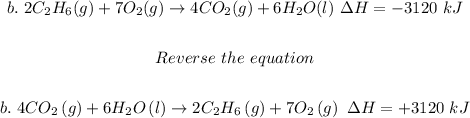
Step 3: (c)
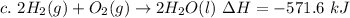
Step 4: Combine (a), (b), and (c) and simplify.
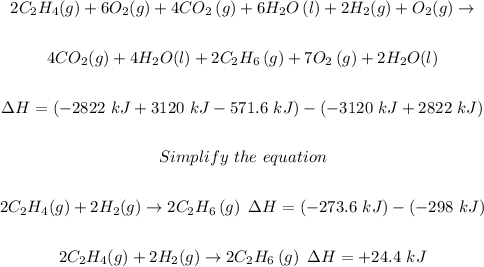
Since the given equation is in 1 mole, then divide through by 2
![\begin{gathered} (2)/(2)C_2H_4(g)+(2)/(2)H_2(g)\operatorname{\rightarrow}(2)/(x)C_2H_6(g)\text{ }\Delta H=(+24.4)/(2)\text{ }kJ \\ \\ C_2H_4(g)+H_2(g)\operatorname{\rightarrow}C_2H_6(g)\text{ }\Delta H=+12.2\text{ }kJ \end{gathered}]()
Therefore, ΔH for the given reaction is +12.2 kJ