Step 1
Molarity can be defined as follows:
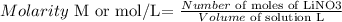
---------------------------
Step 2
Information provided:
0.34 moles LiNO3
Volume = 6.42 L
---------------------------
Step 3
Procedure:
From step1,
Molarity = 0.34 moles/6.42 L
Molarity = 0.053 M
Answer: Molarity = 0.053 M