ANSWER
Step-by-step explanation
Given that;
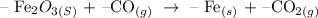
To answer the question below, follow the steps below
Part A
Step 1: Balance the chemical equation of the reaction
The balanced equation of the above reaction is given below as
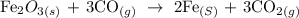
Part B
Calculate the number of moles of iron that can be produced from 1.68 moles of Fe2O3
In the above reaction, 1 mole of Fe2O3 reacts with 3 moles of CO to give 2 moles of iron and 3 moles of CO2
The number of moles of Fe can be determined by using a stoichiometry ratio
Let the number of moles of Fe be x
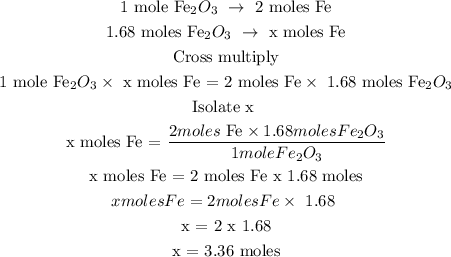
Therefore, the number of moles of Fe is 3.36 moles
Part C
Calculate the amount (in grams) of CO needed to react with 3.02 grams of Fe2O3
Step 1: Find the number of moles of Fe2O3 using the below formula
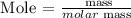
Recall, that the molar mass of Fe2O3 is 159.69 g/mol, and the mass of Fe2O3 is 3.02 grams
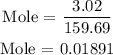
Since the number of moles of Fe2O3 is 0.01891, hence, we can find the number of moles of CO using a stoichiometry ratio
In the above reaction, 1 mole of Fe2O3 will react with 3 moles of CO
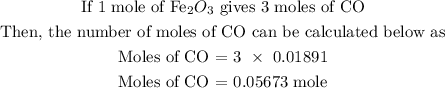
The next step is to find the amount of CO in grams using the below formula
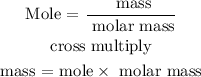
Recall, that the molar mass of CO is 28.01 g/mol
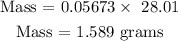
Therefore, the mass of CO is 1.589 grams