Answer:
Percent yield = 16.95%.
Step-by-step explanation:
Let's write the balanced equation:
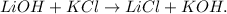
We want to know the theoretical yield of lithium chloride (LiCl), we want to know how much we will produce of this compound from 20 g of lithium hydroxide; so the first step is to convert 20 g of lithium hydroxide (LiOH) to moles using its molar which is 23.9 g/mol (you can calculate the molar mass of a compound using the periodic table). The conversion will look like this:
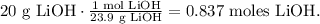
You can see in the chemical equation that 1 mol of LiOH reacted produces 1 mol of LiCl, so the molar ratio between them is 1:1, which means that 0.837 moles of LiOH reacted produces 0.837 moles of LiCl.
The next step is to convert 0.837 moles of LiCl to grams using its molar mass which is 42.3 g/mol:
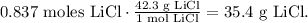
35.4 g LiCl would be our theoretical yield. 6 grams of LiCl that are being produced is the actual yield, so to calculate the percent yield, we use the following formula:
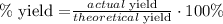
And finally, we replace the given data on it:
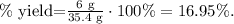
The percent yield is 16.95%.