HNO₃ is a strong acid, while NaOH is a strong base.
They will neutralize themselves by the equation:
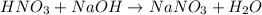
So, both solutions have the same volume, 100 cm, but the base one, NaOH, has a concentration that is greater.
This means that all the acid, HNO₃ will be neutralized by the base and some base will remain in solution.
Since some NaOH will remain not neutralized and it is a strong base, it will dissociate:
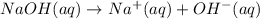
So, the final solution will be basic.