Answer:
266.3 g
Step-by-step explanation:
- __Li + __AlPO₄ → __Al + __Li₃PO₄
The balanced reaction is:
- 3Li + AlPO₄ → Al + Li₃PO₄
First we convert 2.3 moles of AlPO₄ to moles of Li₃PO₄, using the stoichiometric coefficients:
- 2.3 mol AlPO₄ *
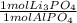 = 2.3 mol Li₃PO₄
= 2.3 mol Li₃PO₄
Then we convert Li₃PO₄ moles into grams, using its molar mass:
- 2.3 mol Li₃PO₄ * 115.79 g/mol = 266.3 g