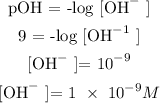ANSWER
![\begin{gathered} [\text{ H}^+\text{ \rbrack = 1}*\text{ 10}^(-5)\text{ M} \\ [\text{ OH}^-\text{ \rbrack = 1 }*\text{ 10}^(-9)\text{ M} \\ \text{ pOH = 9} \end{gathered}]()
Step-by-step explanation
Given that;
pH is 5
Follow the steps below
Firstly, we need to find the H^+ of the sample
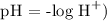
Recall, that pH is 5
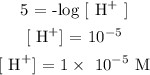
Therefore, [H^+] = 1 x 10^-5 M
Find pOH?
pH + pOH = 14
pH = 5
5 + pOH = 14
subtract 5 from both sides of the equation
5 - 5 + pOH = 14 - 5
pOH = 9
Find OH^- using the below formula