Answer:
71.73grams
Explanations:
Given the chemical reaction;
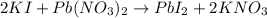
Given the following parameter
Mass of KI = 71.9grams
Determine the moles of KI
According to stoichiometry, 2moles of KI reacts with 1 mole of lead(II) nitrate,the moles of lead(II) nitrate that reacted is given as:
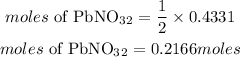
Determine the mass of lead(II) nitrate
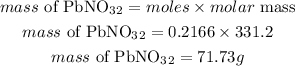
Hence the mass of lead(II)nitrate needed to react completely with 71.9 g of potassium iodide is 71.73grams