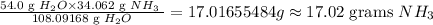Answer
17.02 grams
Step-by-step explanation
Given:
Mass of water = 54.0 grams
What to find:
The grams of ammonia produced.
Step-by-step solution:
The first step is to write a balanced equation for the reaction:
Mg₃N₂ + 6H₂O → 2NH₃ + 3Mg(OH)₂
From the Periodic Table:
Molar mass of water = 18.01528 g/mol
Molar mass of NH₃ = 17.031 g/mol
From the balanced equation above; 1 mol Mg₃N₂ mixed with 6 mol H₂O to produced 2 mol NH₃
In grams; 6 mol x 18.01528 g/mol = 108.09168 g H₂O produced 2 mol x 17.031 g/mol = 34.062 g NH₃
Therefore, 54.0 g H₂O will produce