Answer
Step-by-step explanation
Given that:
The mass of pure silver needed = 800 g
Mass of zinc = 300 g
Mass of silver nitrate = 600 g
What to find:
Will the mass of zinc and silver nitrate be able to make 800 g of pure silver.
Step-by-step solution:
Step 1: Write the balanced equation for the reaction.
Zn + 2AgNO₃ → 2Ag + Zn(NO₃)₂
Step 2: Determine the moles of the reactants.
Using the mole formula, the moles of the reactants will be:
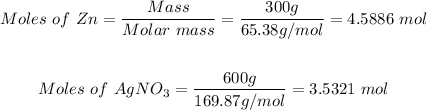
Step 3: Determine the moles of pure silver produced.
Using the mole ratio of Zn to AgNO₃ in the equation and the moles in step 2, we