Answer:
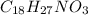
Explanations:
Given the following mass of the elements
Mass of Carbon = 9.72 grams
Mass of Hydrogen = 1.225grams
Mass of nitrogen = 0.631 grams
Mass of oxygen = 13.736 - (9.72+1.225+0.631)
Mass of oxygen = 13.736 - 11.576 = 2.16grams
Convert the mass to moles
Moles of Carbon = 9.72/12 = 0.81moles
Moles of Hydrogen = 1.225/1 = 1.225moles
Moles of Nitrogen = 0.631/14 = 0.0451moles
Moles of Oxygen = 2.16/16 = 0.135moles
Divide by the lowest moles
For Carbon = 0.81/0.0451 = 17.96 = 18
For Hydrogen: 1.225/0.0451 = 27.16 = 27
For Nitrogen: 0.0451/0.0451 = 1
For Oxygen: 0.135/0.0451 = 3
Determine the empirical formula
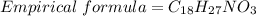
Determine the molecular formula
Hence the molecular formula for capsacin is C18H27NO3