The balanced chemical equation is
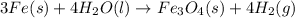
We know that the reaction produces 6 grams of hydrogen gas, this is the actual yield. We need to find the theoretical yield using the stoichiometry of the chemical equation.
According to the equation, 3 moles of Fe produces 4 moles of hydrogen gas, this is the ratio, we know that the molar mass of Fe is 55.845 gr/mol.
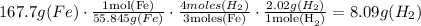
The theoretical yield is 8.09 grams of hydrogen gas. Now we can find the percent yield.
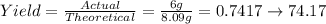
Therefore, the percent yield is 74.17%.