Given:
V1 = 7 mL
V2 = 500 mL
T2 = 525 degrees celsius = 525 + 273 = 798
We know that at STP:
T1 = 0 degrees celsius = 273 K
P1 = 1 atm = 760 torr
We want the final pressure in torr. We have to first convert 1 atm to torr.
1 atm = 760 torr
We also need to convert T from degrees celsius to K
We will use the following equation:
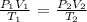
P2 = (760 torr x 7 mL x 798 K)/(500 mL x 273 K)
P2 = 31.10153846 torr