Answer:
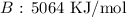
Step-by-step explanation:
Here, we want to get the total bond energy for the products
On the products side, we have 4 C=O bonds formed and 4 O-H bonds formed
We have the values from the given table above
Thus, we have the energy as the sum of the energy of the bonds
Mathematically, we have that as:
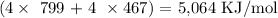
We would be having the answer as negative since the process of bond formation is exothermic (heat is given off and enthalpy change value is negative)