Givens.
• Volume of hexane = 98.0 mL.
,
• Volume of THF = 367. mL.
,
• Final Solution Volume = 456. mL.
To find the volume percent of hexane in this solution, we have to divide the volume of hexane by the final solution volume.
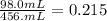
To express it as a percentage, we multiply by 100%.
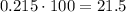
Therefore, the volume percent is 21.5%.