The first step to solve this question is to convert the given mass of copper to moles using its molecular mass (63.5g/mol):
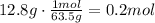
Now, use Avogadro's number to find the number of atoms in 0.2 moles. Remember that it states the number of atoms in 1 mole of substance is 6.022x10^23.
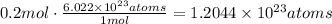
There are 1.2044x10^23 atoms of copper in 12.8g of copper metal.