Answer:
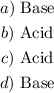
Step-by-step explanation:
Here, we want to determine if each statement is an acid, a base or neutral
a) Donates an OH- ion
In the reaction of an acid with a base, the acid ionizes and donates the hydrogen ion while the base donates the hydroxide ion
What that means is that, it is the base that donates the hydroxide
b) Donates an H+ ion
In the reaction of an acid and a base, it is the acid that donates, the hydrogen ion. The answer here is the acid
c) removes OH- ions from water
An acid releases H+ ions in water and thus takes OH- ions. Thus, it is an acid that removes OH- ions from water
d) Removes H+ ions from water
A base releases OH- ions and thus abstracts H+ ions from water