The molar mass M of a sample is the ratio between its mass m and the amount of substance n that the sample contains:
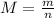
On the other hand, the density ρ of a sample is the quotient between the mass of the sample and its volume V:
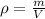
Finally, Avogadro's Number N_A is the number of particles present in 1 mol of a substance. Then, the number of molecules N present in a sample of n mol is:
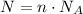
Use these relations to answer the questions.
1)
Isolate n from the first equation and replace the mass of 1.00kg and the molar mass of 175 g/mol to find the amount of substance in 1.00 kg:
![\begin{gathered} n=(m)/(M) \\ =\frac{1.00\operatorname{kg}}{175(g)/(mol)} \\ =(1000g)/(175(g)/(mol)) \\ =5.71\text{mol} \end{gathered}]()
2)
The volume occupied by a sample of mass m is:
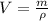
On the other hand, the mass of one molecule can be found writing it in terms of the amount of substance and the molar mass:
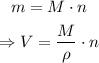
Finally, the amount of substance made up by N molecules is given by:
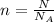
Then, the volume occupied by N molecules of a substance with molar mass M and density ρ is:
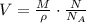
Replace M=175 g/mol, ρ=9300 kg/m^3, N=1 and N_A=6.02*10^23 to find the volume of 1 molecule:
![\begin{gathered} V=\frac{175(g)/(mol)}{9300\frac{\operatorname{kg}}{m^3}}\cdot(1)/(6.02*10^(23)\cdot(1)/(mol)) \\ =\frac{0.175(kg)/(mol)}{9300\frac{\operatorname{kg}}{m^3}}\cdot(1)/(6.02*10^(23)\cdot(1)/(mol)) \\ =3.126*10^(-29)m^3 \end{gathered}]()
3)
Assuming that all particles are arranged as contiguous cubes with the same volume, then the spacing between moecules is equal to the sidelength of those cubes. Since each cube has a volume of 3.126*10^-29 m^3, then the distance between adjacent molecules would be the cubic root of that amount:
![\begin{gathered} L=\sqrt[3]{V} \\ =\sqrt[3]{3.126*10^(-29)m^3} \\ =3.15*10^(-10)m \end{gathered}](https://img.qammunity.org/2023/formulas/physics/college/umrbuab4ss4aqd9i5yu58r3odi8g0hi5t6.png)
Therefore, there are 5.71 mol in 1.00 kg of the substance, the volume occupied by one molecule of the substance is 3.126*10^-29 m^3 and the spacig between adjacent molecules of the substance is approximately 3.15*10^-10 m.