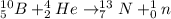Answer:
Step-by-step explanation:
Before balancing the given nuclear reaction, we need to understand what nuclear reaction is.
In simple terms, a nuclear reaction is a process where two nuclei collide to produce one or more new nuclides.
Given the nuclear reaction;
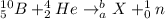
We need to get the unknown element X, the mass number "b" and its atomic number "a"
To get the atomic and the mass number, we will take the sum of the atomic and mass number in the reactant and equate it to the atomic and mass number at the product as shown;
For the atomic number:
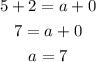
For the mass number:
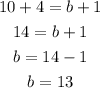
This shows that the required element needed to balance the chemical reaction is an element with an atomic number of 7 and a mass number of 13. The required element will be Nitrogen-13-atom.
The balanced form of the nuclear reaction will be: