Answer:
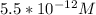
Explanations
The dissociation of zinc sulfide is expressed as:
![ZnS\rightarrow[Zn^(2+)][S^(2-)]](https://img.qammunity.org/2023/formulas/chemistry/college/dg9gf2nb8nihpys3o2dmzf88t79m8fzd9i.png)
The Ksp value is expressed as:
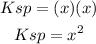
Substitute the given Ksp value to have:
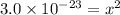
Take the square root of both sides
![\begin{gathered} x=\sqrt{3.0*10^(-23)} \\ x=5.47*10^(-12)M \\ [Zn^(2+)]\approx5.5*10^(-12)M \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/i3ahimdavzqyaoabh3jq5z4uamt084qzpu.png)
Hence the concentration of zinc ions in a saturated zinc sulfide solution is 5.5 * 10^-12M