[a] In the reaction, you can see that 4 moles of lithium nitrate (LiNO3) produce 2 moles of lithium sulfate (Li2SO4). The first thing that we need to do, is found the moles of lithium sulfate based on its mass (200.0 grams) and then, find the number of moles of lithium nitrate produced (remember that the molar mass of Li2SO4 is 110 g/mol).
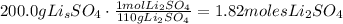
Now, doing a rule of three to find the number of moles of LiNO3, that would be:
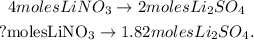
The calculation to find the number of moles of LiNO3 would be:
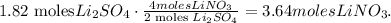
Using this value and the molar mass of LiNO3 which is 69 g/mol, we're going to obtain the mass in grams of LiNO3, like this:
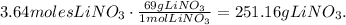
We will need 251.16 grams of lithium nitrate to make 200.0 grams of lithium sulfate.
[b] You can see that we're producing 3.64 moles of LiNO3. In the reaction, 4 moles of LiNO3 are reacting with 1 mole of Pb(SO4)2 and we will take into account this information because LiNO3 is the reactant that runs out first; the calculation to find the number of moles of lead (VI) sulfate would be:
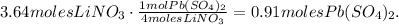
Using this value and the molar mass of Pb(SO4)2 which is 399.1 g/mol, we're going to obtain the mass of Pb(SO4)2:
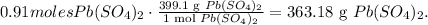
We will need 363.18 grams of lead (IV) sulfate.