ANSWER
Calcium atom has two electrons to contribute to the sea of delocalized electrons
Step-by-step explanation
Calcium belongs to group two metals with the atomic number of 20
The electronic configuration of calcium is written below as
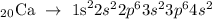
In the above configuration, calcium has two electrons at its outermost shell. This means it valence electrons is 2
Therefore, calcium is readily to lose the two electrons two attain it octet configuration.
Hence, calcium atom has two electrons to contribute to the sea of delocalized electrons