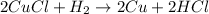To balance the the following equation we will take the following steps:
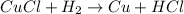
-We will balance the the hydrogen on the product side to be equivalent with that on the reactant side of the equation. The number 2 will go infront of HCl.
-Now that we have two chlorines on the product side, we will balance the chlorines on the reactant side and so the number 2 will go in fron of the CuCl. We also need to add a 2 to to the Cu on the product side. The balanced equation will be: