ANSWER
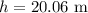
Step-by-step explanation
Since the temperature is constant, applying the ideal gas equation, we have that the product of volume and pressure remains constant:
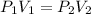
When they are submerged in water, their pressure is:
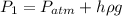
where Patm = atmospheric pressure
h = depth below the surface
ρ = density
g = acceleration due to gravity
At the surface of the water:
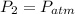
Applying the ideal gas equation, we have that:
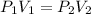
But we have that V2 = 3V1. Substituting that into the equation:
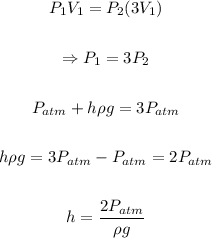
Substitute the given values into the equation and solve for h:
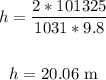
That is the height when the bubbles are released.