The first thing we need to do is balance the equation. For that, we count the atoms of each element on each side of the reaction.
We have 2 hydrogen atoms and two oxygen atoms in the reactants. And two atoms of hydrogen and one of oxygen in the products, so we must balance the oxygens by placing the coefficient two in the H2O molecule.
We adjust the moles of hydrogen by placing coefficient two on the H2 molecule in the reactants. So the balanced equation is:
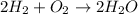
Now, we must find the moles of each of the reactants. We divide the given masses by their molar mass.
Moles of H2
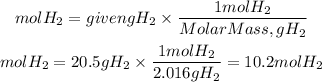
Moles of O2
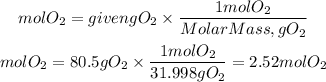
Now, we need to find the limiting reactant. The limiting reactant is the reactant that will produce the least amount of product.
One way to determine the limiting reactant is by dividing the moles found by the stoichiometric coefficient of each compound. The one with the smallest quotient will be the limiting reactant.
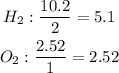
The limiting reactant will be oxygen, so we will do the calculations according to this reactant.
The ratio of H2O to O2 is 2/1. So the moles of O2 will be:
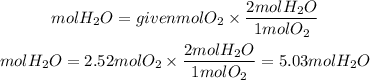
The grams of H2O will be:
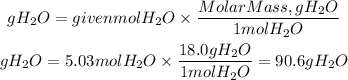
Answer: It will be produced 90.6 grams of H2O