So,
We could state the following equations:
Let "x" be the number of visits of a person in a trip, and let "y" be the cost.
For plan A, we could write:
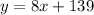
For plan B, we could write:
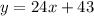
To find the number of visits that a person must make for plan A and plan B to be in equal value, we should equal the equations of both plans, and solve the resulting equation for x, as follows:
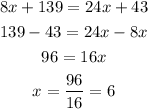
Therefore, x=6.
So, if a person makes 6 visits, plan A and plan B would be in equal value.