Answer
453.9 kPa
Step-by-step explanation
Given:
Initial volume, V₁ = 750.0 mL
Initial pressure, P₁ = 151.3 kPa
Final volume, V₂ = 250.0mL
What to find:
The new pressure when the volume is decreased to 250.0 mL.
Step-by-step solution:
The new pressure when the volume is decreased to 250.0 mL can be calculated using the Boyle's law formula below
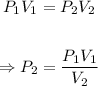
Plugging the values of the given parameters into the formula, we have
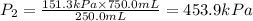
The new pressure when the volume is decreased to 250.0 mL is 453.9 kPa
: