We are asked to determine the amount of energy required to turn ice into water and then that water to boil completely.
First, we calculate the energy required to turn ice into water at 0°C. We will use the following formula:
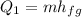
Where "m" is the mass and hfg is the heat of fusion which is a constant equivalent to 334 kJ/kg. Replacing the values we get:
![Q_1=(0.350\operatorname{kg})(334\frac{kJ}{\operatorname{kg}})]()
Solving the operation:
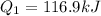
Now we determine the amount of heat required to convert the water at 0°C into water at 100°C which is the boiling point. We will use the following formula:
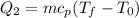
Where "cp" is the specific heat of water which is a constant equivalent to 4.18 kJ/kg°C. Replacing the values:
![Q_2=(0.350\operatorname{kg})(4.18(kJ)/(kgC))(100^0C-0^0C)]()
Solving the operation
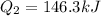
Now we determine the amount of heat required to turn water at 100°C into steam. To do that we will use the following formula:
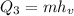
Where "hv" is the heat of vaporization and is equivalent to 2260 kJ/kg. Replacing we get:
![Q_3=(0.350\operatorname{kg})(2260\frac{kJ}{\operatorname{kg}})]()
Solving the operations:
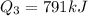
Now we add all the energy to determine the total energy required:
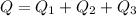
Replacing the values:
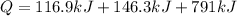
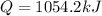
Therefore, the total amount of heat is 1054.2 kJ.