To find the number of molecules present in 0.40 moles of water (H2O), we have to use Avogadro's number which is 6.02 x 10^(23). Remember that this number represents the number of molecules or atoms per mole of a compound.
The calculation is:
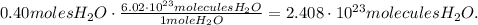
The answer is that 0.40 moles of H2O contain 2.408 x 10^(23) molecules of H2O.