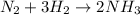
Firstly we convert the mass of nitrogen gas into moles:
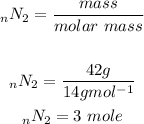
Based on the chemical equation 1 mole of nitrogen gas produces 2 moles of ammonia. We can set up an equation to determine how many moles of NH3 will be produceds after reacting with 3moles of nitrogen. The value we get we will convert it to mass.
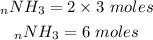
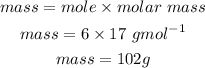
Mass of NH3 produced is 102g.