To answer this question we have to use ideal gas law:

Where P is the pressure of the gas, V is its volume, n is the number of moles of the gas, R is the ideal gas constant (0.082atmL/molK) and T is the temperature. Use this formula to find the volume by solving for V and replacing for the given values, remember that STP is standar temperature and pressure, which are 273.15K for temperature and 1atm for pressure:
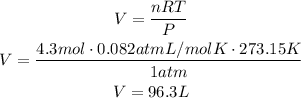
According to this, we have 96.3 liters of gas.
The correct answer is the second choice.