Step 1
In a titration, the next equation is used in the equivalence point:
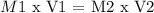
Where,
Number 1 will the conditions of HBr
Number 2 will the conditions of Ca(OH)2
----------------------
Step 2
Information provided:
M1 = 0.0105 M
V1 = unknown
---
M2 = 0.0100 M
V2 = 125 mL
-----------------------
Step 3
Procedure:
From step 1, V1 is found as follows:
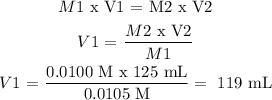
Answer: V1 = 119 mL