Answer:
0.0885M
Explanations:
In order to determine the concentration of the KOH used in this lab, we will use the dilution formula expressed as
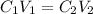
where:
• C₁ and C₂ are the ,initial and final concentrations
,
• V₁ and V₂ are the ,initial and final volumes
Given the following parameters
• C₁ = 0.138M
,
• V₁ = 25.0mL = 0.025L
,
• V₂ = 39.0mL = 0.039L
Required
Final concentration C₂
Substitute the given parameter into the formula to have:

Hence the concentration of the KOH used in this lab is 0.0885M