SOLUTION
The volume of a gas varies inversely with the pressure
This is written as:
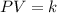
Where k is a constant
When V=2L, P=1165kPa
Substitute these values into the above equation
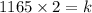
It follows:
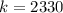
Substituting the value of k into the equation gives
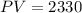
Find V when P=466kpa
Substitute P=466 into the equation PV=2330
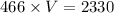
Solve for V
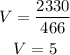
Therefore The required volume is 5L