A concentration in mass percent is, by definition, the amount of mass os solute that is contained in 100g of solution, expressed in terms of percentage. We can find this by the following formula:
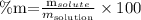
In this case, the mass of solute (sodium) is 48mg, i.e., 0.048g, and the mass of solution would be 288 + 0.048 = 288.048g (water + solute). We can now substitute these values on the equation above:
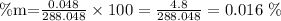
The concentration of sodium in the soft drink is, in mass percent, 0.016%