First, we must know this:
1 mole of anything = 6.022 x 10^23 6.022 formula units
Procedure:
We have 9.11 moles, so, we do this:
1 mole of Na2SO4 ------------ 6.022 x 10^23 6.022 formula units
9.11 moles of Na2SO4 ------------ x
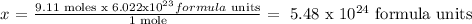
Answer: 5.48x10^24 formula units