Answer:
+2. Option A is correct.
Explanations:
Given the compound FeSO₄, we are to get the charge on Iron(Fe). From the given compound;
• The o,xidation state ,of sulfur (S) is +6
,
• Oxidation state of oxygen is -2(4) = -8
Let the oxidation state of iron (Fe) be "x"
Taking the sum of the oxidation state and equate to zero to get the value of "x"
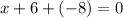
Simplify the result
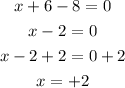
This shows that the charge on iron in FeSO4 is +2