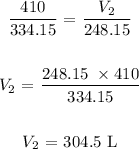Answer:
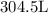
Step-by-step explanation:
Here, we want to get the volume of the nitrogen gas at the lower temperature
From Charles' law, we know that volume and temperature (in Kelvin) are directly proportion
The mathematical relationship is:
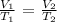
Where:
V1 is the initial volume which is 410 L
V2 is the final volume which is unknown
T1 is the initial temperature which we will convert to Kelvin by adding 273.15 K, we have it as 61 + 273.15 = 334.15 K
T2 is the final temperature which we have to convert to Kelvin by adding it to 273.15K : We have that as -25 + 273.15 = 248.15 K
Substituting the values, we have it that: