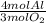1) Molar ratio. This number shows the amounts in moles of the compound in a reaction. We can also say that this number shows how many moles of the product are formed from a specific reactant.
2) Balance the chemical equation.
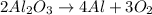
3) Molar ratios
The molar ratio of aluminum oxide (Al2O3) to aluminum (Al).
Al2O3 = there is a 2 in front of this formula.
Al = there is a 4 in front of this formula.
The molar ratio of these two compounds is 2 mol Al2O3: 4 mol Al.
As a fraction
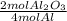
The molar ratio of aluminum oxide (Al2O3) to oxygen (O2).
Al2O3 = there is a 2 in front of this formula.
O2 = there is a 3 in front of this formula.
The molar ratio of these two compounds is 2 mol Al2O3: 3 mol O2.
As a fraction
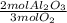
The molar ratio of aluminum (Al) to oxygen (O2).
Al = there is a 4 in front of this formula.
O2 = there is a 3 in front of this formula.
The molar ratio of these two compounds is 4 mol Al: 3 mol O2.
As a fraction