a) Strontium has 38 electrons.
Its electronic configuration is,
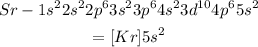
As it can be seen, the valency of strontium is 2. Thus it needs to lose 2 electrons to be stable.
Thus the answer is '2 lost'.
b) The atomic number of antimony is 51. The electronic configuration of antimony (Sb) is
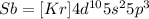
Thus The