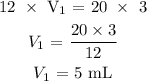Answer:
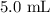
Step-by-step explanation:
Here, we want to get the volume needed to make 20 mL of 3 M HCl
Mathematically, according to the dilution formula:
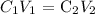
Where C1, V1 are the initial molarity and volume respectively, while:
C2, V2 are the final molarity and volume respectively
Substituting the values, we have it that: