So,
First of all, we should remember what the solubility rules are.
Let me upload an image for this:
The following complete reaction is given in our problem:
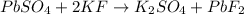
We're going to analyze each compound and check its solubility.
Let's begin with PbSO4:
If you see the rule number 4, all compounds containing sulfate (SO4) are soluble, except those of lead (Pb).
So, the compound PbSO4 is not soluble in water.
Let's see KF:
If you see the rule number 1, all common salts of the group 1A elements are soluble.
So, KF is soluble in water.
Let's see K2SO4 now:
If you look at the rule number 4, all compounds containing sulfate (SO4) are soluble. Since "K" doesn't belong to the exceptions group, we can say that:
K2SO4 is soluble in water.
And finally, let's analyze the compound PbF2:
If we notice, all Fluorides are soluble except if those of lead, so,
PbF2 is not soluble in water.