Answer
Percent yield for the reaction = 32.86%
Step-by-step explanation
Given:
Moles of NO = 7.0 mol
Moles of O₂ = 5.0 mol
Moles of NO₂ generated = 2.3mol
2 NO (g) + O₂ (g) → 2 NO₂ (g)
What to find:
The percent yield for the reaction.
Step-by-step solution:
The percent yield for the reaction can be calculated using:
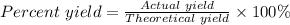
The actual yield = moles of NO₂ generated = 2.3mol
The theoretical yield can be calculated as follows:
First, we need to determine the limiting reactant, using balanced equation for the reaction.
From the equation, 2 mol NO reacts with 1 mol O₂
So 7.0 mol NO will require (7.0 mol x 1 mol)/2 mol = 3.5 mol O₂
This means the 7.0 mol of NO will be completely consumed by only 3.5mol of O₂. Hence, NO is the limiting reactant and O₂ is the reactant in excess.
The next step is to use the limiting reactant to calculate the theoretical yield for the reaction.
From the reaction equation, 2 mol NO generates 2 mol NO₂
So 7.0 mol NO will generate:
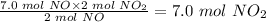
Finally, put actual yield = 2.3 mol, and theoretical yield = 7.0 mol into the percent yield formula above:
