Step-by-step explanation:
According to the next equation,
2Li + Cl₂ → 2 LiCl (completed and balanced)
-------
The oxidation state of each element changes as follows:
Li) goes from 0 to +1
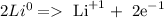
Cl) goes from 0 to -1
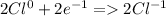
(these equations above are the half-reactions)
Therefore,
Answer:
Lithium undergo oxidation
Chlorine undergo reduction