Given the number of atoms or molecules, we need to use Avogadro's number, but first, let's review this concept: The number 6.02×10^(23 ) is called Avogadro's number, the number of representative particles in a mole or atom.
Remember that the symbol of tellurium is Te. Now, doing the conversion to moles we're going to obtain:
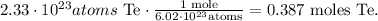
With this number, we can obtain the grams of tellurium using the molar mass that you can find in the periodic table which is 127.6 g/mol. The conversion from moles to grams will be:
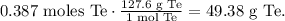
So, 2.33 x 10^(23) atoms of Te are 49.38 of tellurium.