Answer:
The volume is 18,000mL.
Step-by-step explanation:
The given information from the exercise is:
- C2H6 gas
- Mass: 30.0g
- Pressure: 960mmHg
- Temperature: 275K
1st) To calculate the volume it is necessary to use the Ideal Gas formula, making sure that the variables are in atm, liters, mole and Kelvin.
- Conversion of 960mmHg to atm:
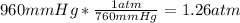
- Conversion of 30.0g to moles, using the molar mass of C2H6 (30g/mol):
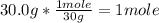
The number of mole is 1.
2nd) Now we can replace the values in the Ideal Gas formula to find the volume:
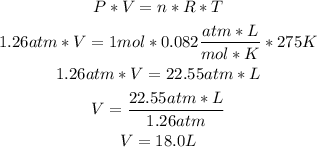
3rd) Finally, we have to convert the liters to mL:
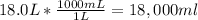
So, the volume is 18,000mL.