Step 1 - Reading and understanding the chemical equation
The provided chemical equation is:
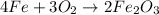
We can read this equation as follows:
4 moles of Fe react with 3 moles of O2 thus producing 2 moles of Fe2O3
As the exercise is specifically asking about the proportion between the reactants, we can further simplify this statement to:
4 moles of Fe react with 3 moles of O2
Step 2 - Converting the moles proportion to grams proportion
We can easily convert between moles and grams by using the molar mass of each substance (56 g/mol for Fe, 32 g/mol for O2):
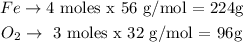
We can rewrite the proportion thus as:
224 of Fe react with 96g of O2
Step 3 - Calculating the needed amount of O2
Now we just have to set a proportion. We know 125g of Fe were used, so:
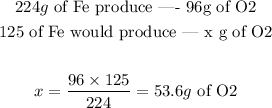
Answer: 53.6g of O2