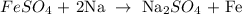Answer:
Step-by-step explanation:
Using the activity series, we want to predict the products of the reaction
From the series, we know that sodium is higher than iron. This means that sodium can displace iron from its salt
Thus, we have the equation of reaction as: