Answer: Volume = 333.33 ml.
Explanations :
GIVEN :
• Volume ,(V1)= 100ml
• Concentration ,(C1) = 2.50 M
• Concentration ,(C2) = 0.75 M
• Volume ,(V2) = ?
The relationship between Volume and Concentration can be represented by formula :
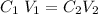
Replacing the given parameters into the formula above , Final Volume (V2) will be :

This means thea final volume = 333.33ml