Given:
The mass of the block of pure metal is: m = 250 kg
The change in the temperature is: ΔT = 66.0 °C - 20.0 °C = 46 °C
The heat supplied to the material is: Q = 5.35 kJ
To find:
The specific heat.
Step-by-step explanation:
The expression the heat supplied/released Q, the mass of the material m, the specific heat C, and the change in temperature ΔT is given as:
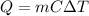
Rearranging the above equation, we get:
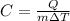
The heat can be converted from Kilojoules to kilocalories as:
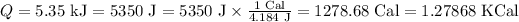
Substituting the values in the above equation, we get:
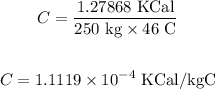
Final answer:
The value of specific heat is 1.1119 × 10⁻⁴ KCal/kg °C.